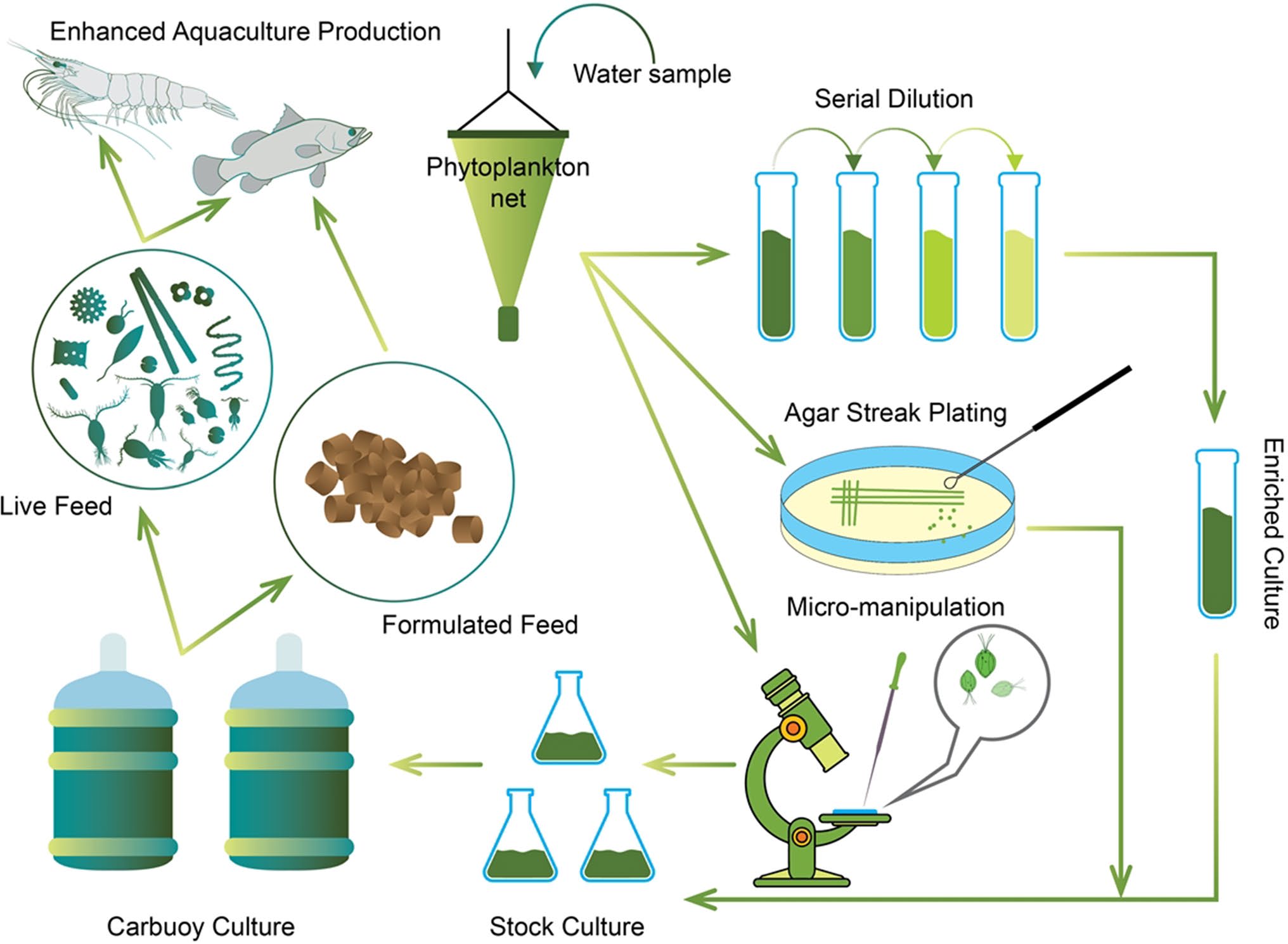
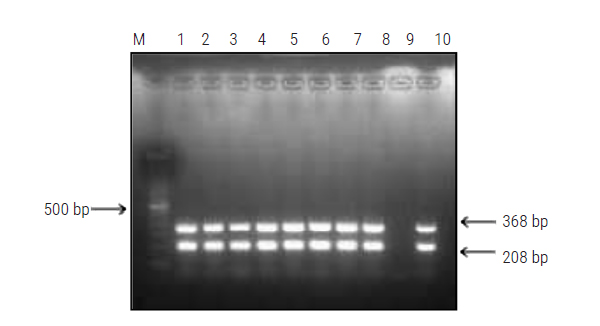
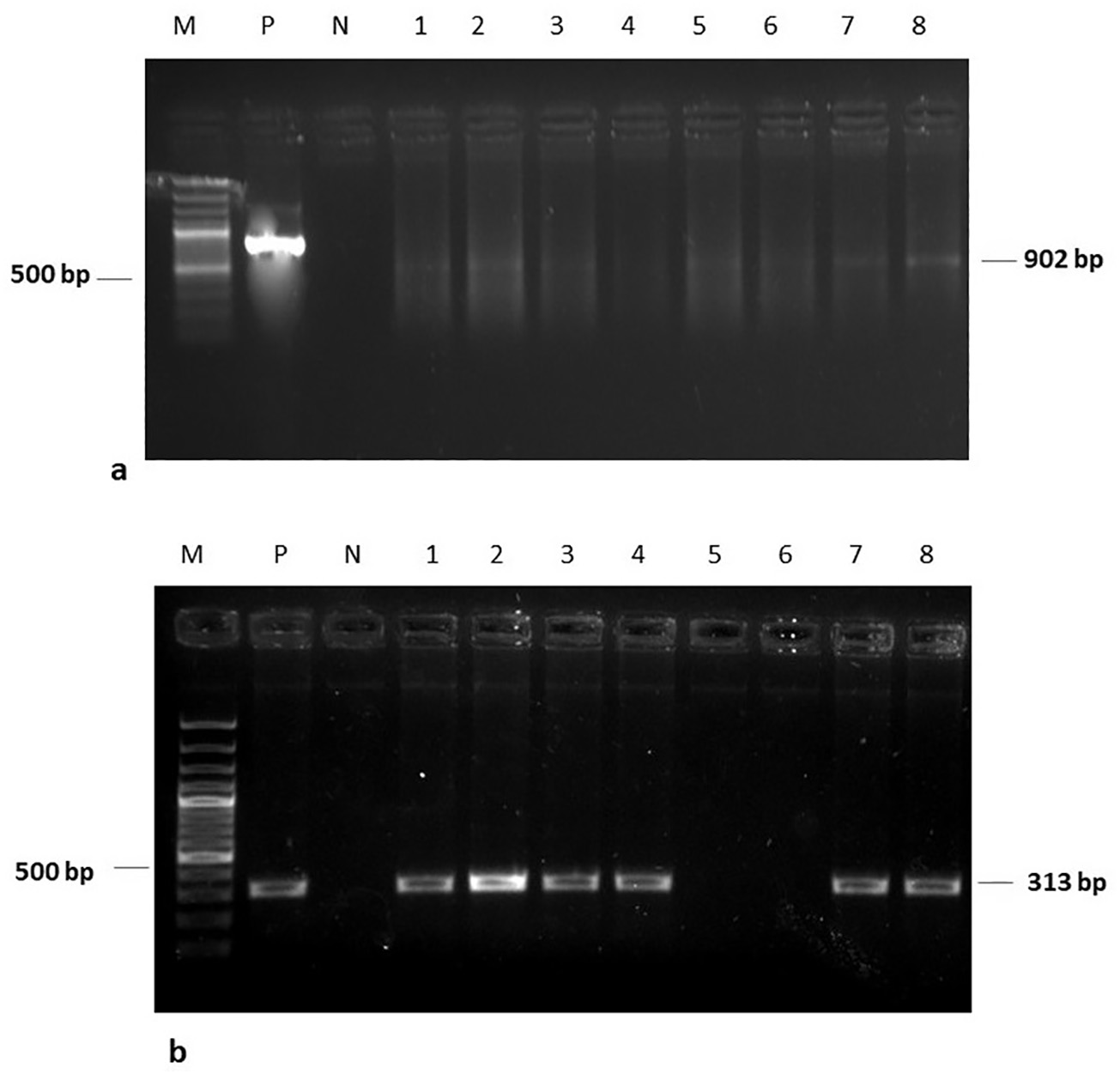
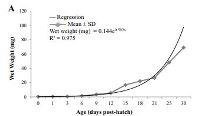
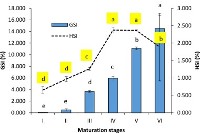
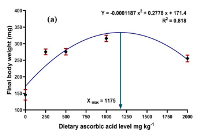
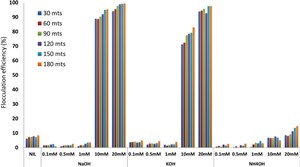
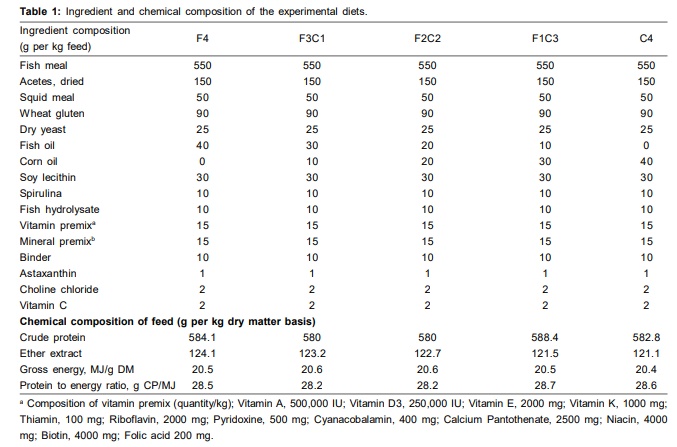
Latest Updates
ABOUT THE INSTITUTE
Indian Council of Agricultural Research (ICAR), New Delhi, under the Ministry of Agriculture, Government of India established the Central Institute of Brackishwater Aquaculture (CIBA) on 01-04-1987. The Central Institute of Brackishwater Aquaculture serves as the nodal agency for the development of brackishwater aquaculture in the country. The Headquarters of the Institute is located at Chennai with an experimental field station at Muttukadu (MES), about 30 km south of Chennai.
RESEARCH UPDATES
Sustainable intensification and transformation of India’s brackishwater aquaculture in response to changing scenario, is critical for global seafood availability, nutritional security and in turn trade revenues for the country.